The rise of new anti-cancer forces! CAR-T therapy for four major solid tumors brings unprecedented survival breakthrough.
Immunotherapy has become a trump card in the fight against cancer! By stimulating the ability of our immune system to fight cancer, it sets up a brand-new principle for cancer therapy, which can be said to be a milestone in the process of human struggle against cancer!
In addition to the well-known immune checkpoint inhibitors, CAR-T therapy is undoubtedly the leader in the field of global cancer cell immunotherapy. Because of the successful research and development of CAR -T therapy, it has created a miracle of "curing" cancer. This treatment method has been proved to induce significant reactions-even patients with advanced cancer whose survival time is only a few months can be completely eradicated, and in some cases the strong response lasts for months or even years. For example, Emily, a leukemia girl, was dying after being diagnosed at the age of 5, but now she has been successfully cured by CAR-T cell therapy for nearly 10 years, and she has become the spokesperson of this epic therapy and has been recorded in history.

Emily, a leukemia girl, declared cancer-free for 9 years.
CAR-T therapy brings changes to global cancer treatment.
In recent years, there has been a worldwide upsurge in the research and development of CAR-T therapy, because whoever makes CAR-T therapy technology with better curative effect and lower side effects will undoubtedly take the lead in eating the first crab and open a new era of tumor immunotherapy. At present, six CAR-T therapies have been approved by FDA, and two CAR-T products have been listed in China, and seven CAR-T therapies have been approved worldwide!

Unfortunately, CAR-T therapy is only approved in hematological tumors at present, because the tumor cells of hematological tumors have ancestral targets, such as CD19 (only existing in tumor cells but not in normal cells), and we can easily rely on this target to lead CAR-T cells to find cancer cells and eliminate cancer.
However, there is no such obvious target in solid tumor that exists only in cancer cells but not in normal cells. Therefore, the clinical efficacy of CAR-T cells in the treatment of solid tumors has been poor. The medical community has always hoped that CAR T cells can develop new specific targets for more solid tumors!
The rise of new anti-cancer forces! Six solid tumor CAR-T therapies bring unprecedented survival breakthrough!
At present, with the change of CAR-T algebra, CAR-T has obviously improved in proliferation and cytokine release, and this technology has finally broken the ice. More and more clinical trials have begun to try to use CAR-T in the treatment of solid tumors, and patients with advanced solid tumors are welcoming warm spring!
Representative antigen targets include:
Mesothelin for the treatment of mesothelioma, pancreatic cancer, ovarian cancer and lung cancer;
CEA is used to treat lung cancer, colon cancer, gastric cancer, breast cancer and pancreatic cancer;
MUC-1 is used for treating liver cancer, lung cancer, pancreatic cancer, colon cancer and gastric cancer;
GPC3 for the treatment of liver cancer;
EGFRvII is used to treat glioma and head and neck tumors;
B7-H3, used for treating Ewing sarcoma, rhabdomyosarcoma, nephroblastoma, neuroblastoma and medulloblastoma, as well as brain stem tumor (DIPG) which is particularly difficult to treat;
PSMA, used for prostate cancer, etc.
Claudin18.2, used for gastric cancer, pancreatic cancer, etc.
….
Application of 01GPC3 CAR-T in liver cancer
Liver cancer is the second leading cause of cancer death in the world (accounting for 8.2% of the total). Although great progress has been made in diagnosis and treatment in recent years, the effect of current targeted and immunotherapy is limited, and better treatment schemes are urgently needed in clinic.
GPC3 is a carcinoembryonic antigen, which is involved in cell proliferation, differentiation, migration and apoptosis. What excites researchers is that GPC3 protein is almost not expressed in 21 normal tissues such as heart, liver, spleen, lung and kidney, but it is expressed in 70-80% hepatocellular carcinoma and lung squamous cell carcinoma. GPC3 is considered as a promising tumor immunotherapy target because of its tumor specificity. The good news is that there are monoclonal antibodies, double antibodies, therapeutic vaccines and GPC3 CAR-T therapy for this target, which have shown great potential in liver cancer.
In 2020, good news came from the Chinese team: the results of Phase I clinical research on the treatment of advanced hepatocellular carcinoma (HCC) with the second generation CAR-T cell therapy targeting GPC3 were released, and the research results were published in the international heavy oncology journal Clinical Cancer Research, which caused a sensation. It is worth mentioning that this is the world’s first clinical trial targeting GPC3 CAR T cells to treat hepatocellular carcinoma, and the research results of Chinese scientists once again shine on the world stage.
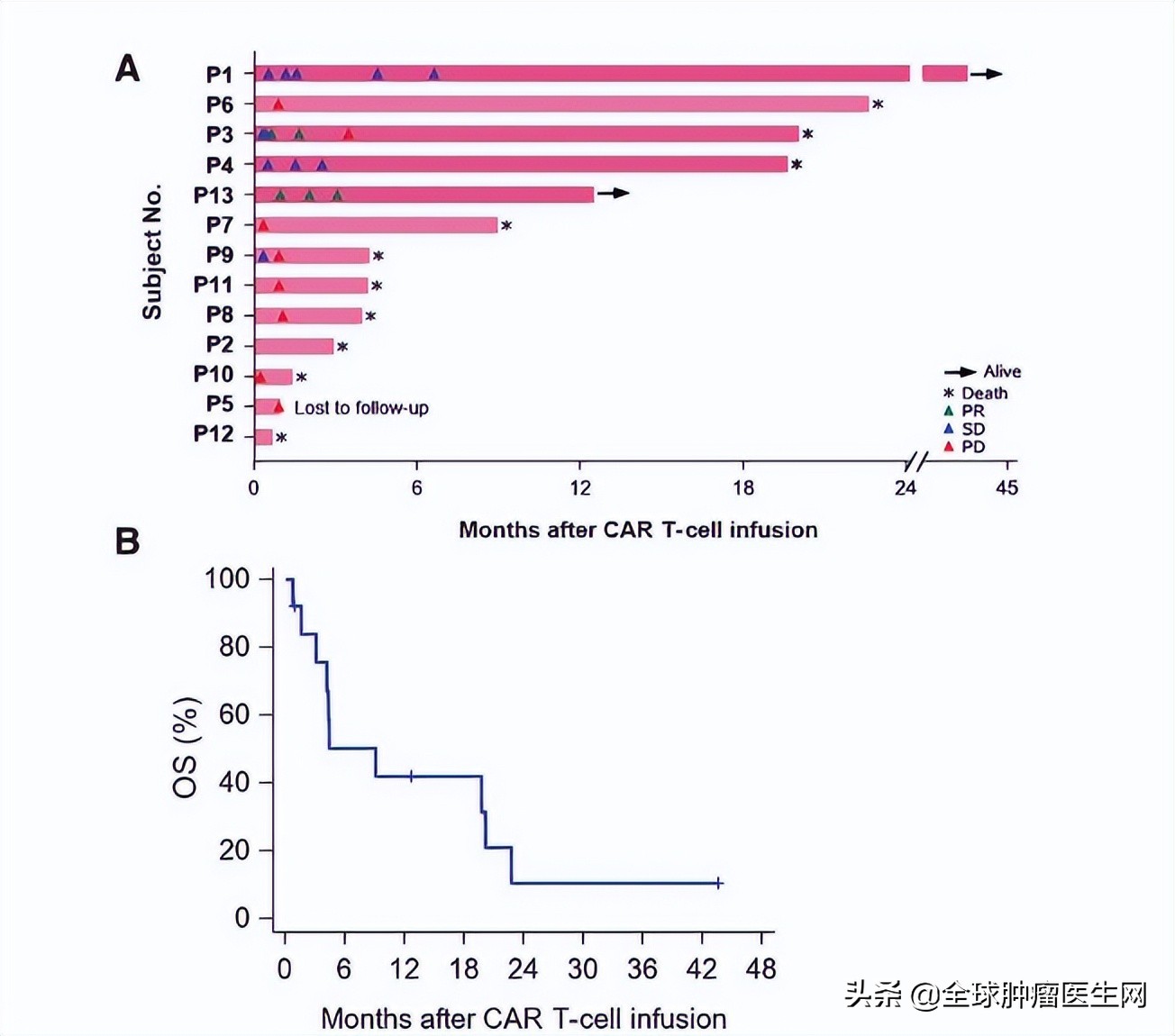
In this newly published study, 13 patients with advanced liver cancer were enrolled (from December 2015 to July 24, 2019).
The results show that:
Of the 13 subjects, 2 obtained partial remission (PR);
The 3-year, 1-year and 6-month survival rates were 10.5%, 42.0% and 50.3% respectively.
The median survival time (OS) was 278 days (95% CI: 48,615 days).
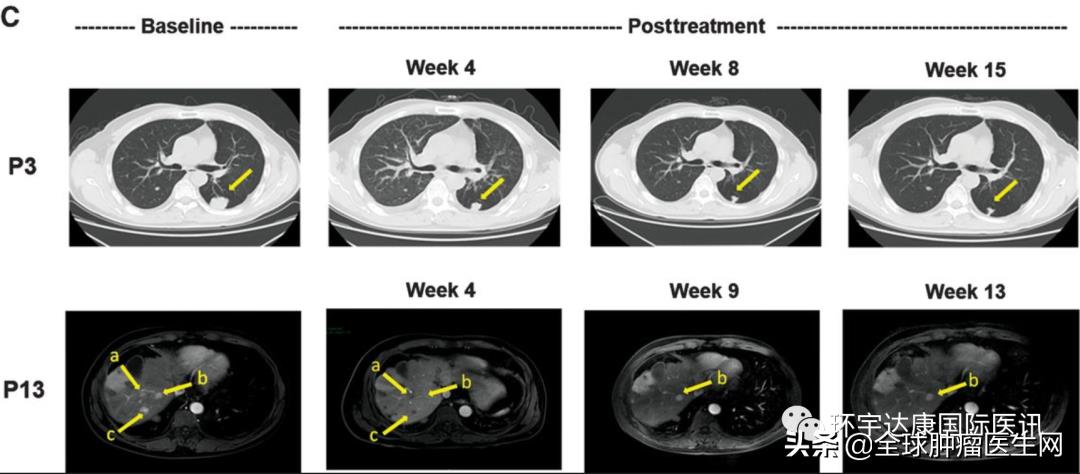
Two patients with advanced liver cancer, numbered 3 and 13, were very lucky. After receiving GPC3 CAR T treatment, the lesions were significantly reduced, and some of the target lesions even subsided!
The good news is that the new T-therapy has officially started to recruit patients in China. At present, several well-known cancer hospitals in China have taken the lead in clinical research, and some patients have successfully entered the group through the application of the global oncologist network.
In addition, at the virtual annual meeting of the American Society of Clinical Oncology (ASCO) in 2021, CARsgen Therapeutics presented a new fourth-generation chimeric antigen receptor T (CAR-T) cell ORI-CAR-001 (NCT03980288) based on the second-generation CAR-T.
The fourth generation of CAR-T cells can secrete transgenic cytokines when targeting CAR signal transduction in tumor tissues after additional engineering modification. Therefore, in addition to direct anti-tumor attacks, they can also trigger T cells to eliminate antigen-negative cancer cells in the target site, which is more effective!
The preliminary results of this study show that the safety and effectiveness of Ori-CAR-001 in patients with GPC3 positive recurrent/refractory HCC are encouraging.
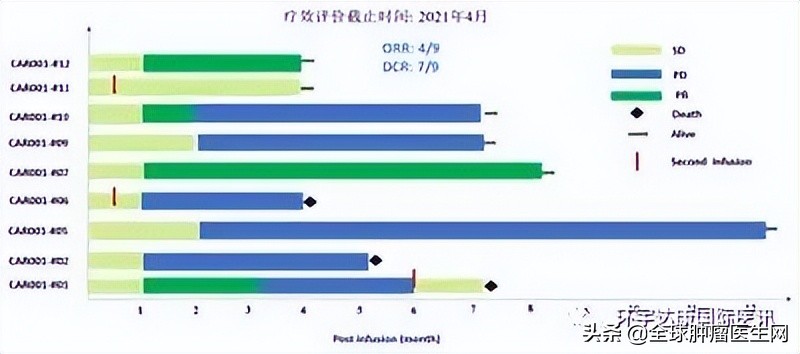
By March 10th, 2021, 11 patients had participated in this study. All subjects were advanced hepatocellular carcinoma, and previous treatment methods (including chemotherapy, TACE and targeted therapy) failed.
The results showed that among 9 evaluable subjects, 4 achieved partial remission (PR), 3 achieved disease stability (SD) and 2 developed disease progression (PD). The objective remission rate was 44.4%, and the disease control rate reached 77.8%.
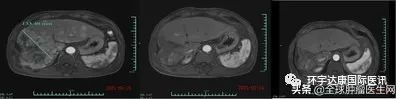
It is worth mentioning that the tumor volume of subject 007 decreased by more than 80% one month after cell therapy infusion! Subject 012 also saw a good tumor response. He had advanced and diffuse large HCC and joined the study after radiotherapy, targeted drugs and 12 TACE failures. On the 28th day after CAR-T cell infusion, the diameter of the target tumor lesion decreased from 133 mm at baseline to 9 mm, a decrease of more than 93%. At present, the subject is being evaluated at the third month after CAR-T cell infusion, and MRI scan shows that the tumor has almost completely subsided!
02
Application of claudin18.2 CAR-T in gastric cancer and pancreatic cancer
Claudin 18.2 is a target of pantumor, which is expressed in many epithelial tumors, especially in gastric cancer and pancreatic cancer. In gastric cancer or gastroesophageal junction cancer, the high expression of Claudin 18.2 was detected in as many as 60% patients. Based on this, researchers in China developed the first CAR-T cell therapy for Claudin18.2 –CT041.
The preclinical research results of CT041 cells in the treatment of gastric cancer show that gastric tumors can be completely eliminated in mouse models without off-target toxicity.
Based on excellent preclinical data, China took the lead in developing a multi-center, open-label phase I clinical study of Claudin 18.2 CAR-T cells in the treatment of patients with digestive system tumors, including dose escalation stage and dose expansion stage. The main purpose was to evaluate the safety and tolerance of CT041, and the secondary purpose was to evaluate the efficacy and pharmacokinetics.
It is worth mentioning that all the patients in the group have received second-to third-line treatment, Claudin 18.2 is moderately highly expressed, most of them have multiple organ metastases, and nearly half (42.9%) of them have also received PD-1 treatment, which is extremely difficult to treat clinically.
Experimental design:
By April 8, 2021, 37 patients had received CT041 infusion and completed the evaluation for at least 12 weeks, including 28 cases of gastric cancer/gastroesophageal junction cancer (GC/GEJ), 5 cases of pancreatic cancer (PC) and 4 cases of other digestive system tumors. The dose levels of CAR-T cells were 2.5× 10, 3.75× 10 and 5.0× 10, respectively.
The results show that:
The target lesions of 31 patients were reduced in different degrees. The overall objective remission rate (ORR) was as high as 48.6%, and the disease control rate (DCR) was 73.0%.
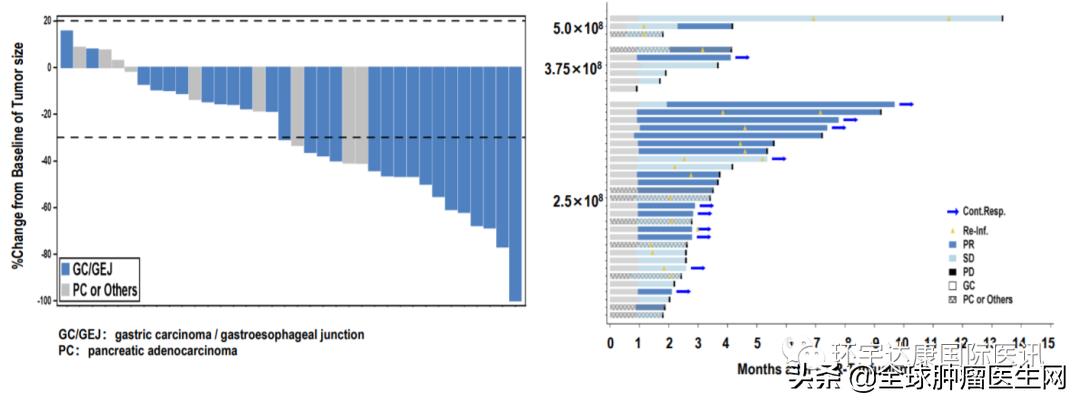
Source CARsgen Therapeutics
Among them, the objective remission rate (ORR) and disease control rate (DCR) were as high as 61.1% and 83.3%, respectively, in 18 patients with gastric/gastroesophageal junction cancer who failed in at least second-line therapy (including 8 patients who had received PD-1) at the dose of 2.5× 10 CAR-T cells. MPFS is 5.6 months and mOS is 9.5 months.
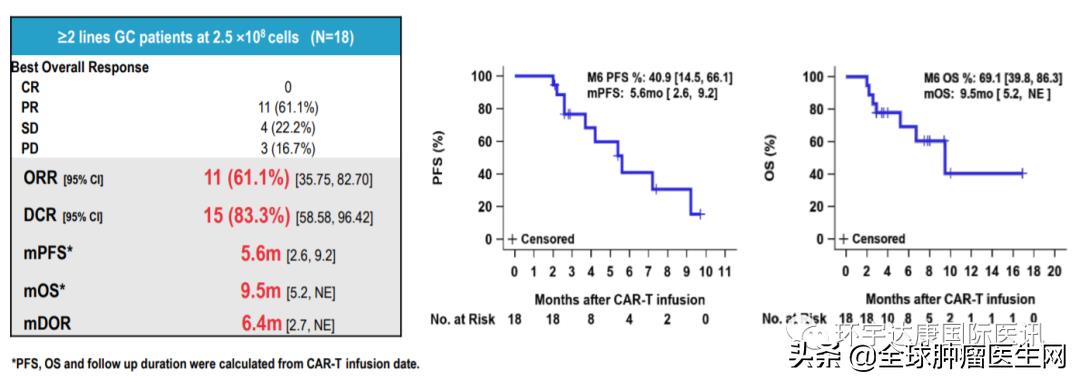
Source CARsgen Therapeutics
In terms of safety, CT041 is generally well tolerated. No treatment-related deaths or immune cell therapy-related neurotoxic syndromes (ICANS) were reported. About 95% patients experienced CRS, all of which were Grade 1 or Grade 2.
The reason why domestic experts agree that this data is excellent is because historical data show that the effective rate of chemotherapy is only 4% ~ 8% and the effective rate of anti-PD-1 antibody is only 11% for patients with gastric/gastroesophageal junction cancer who failed at least the second-line treatment in the past, and the objective remission rate of CT041 for second-line treatment failure, even after PD-1 treatment failure, can be as high as 61.1% (which means 61.1% in the late stage)
In addition to the above data, the treatment of two patients who achieved objective remission was also announced:
01
Patient 04, 53-year-old male.
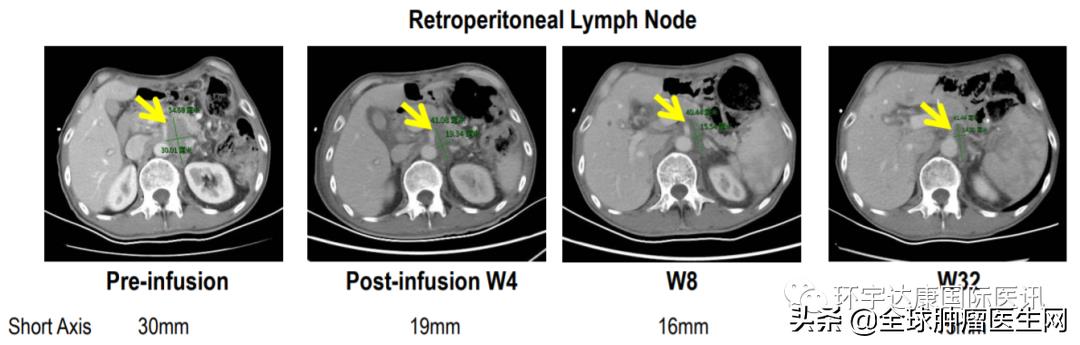
Source CARsgen Therapeutics
Advanced gastric cancer with liver, lung, bone metastasis and multiple lymph node and peritoneal metastasis has received two kinds of systemic therapy, including PD-1 antibody, CLDN was 18.260% (3+). After CAR-T therapy, the tumor shrank by nearly 50% and remained in remission for 32 weeks.
02
Patient 08, 57-year-old female.
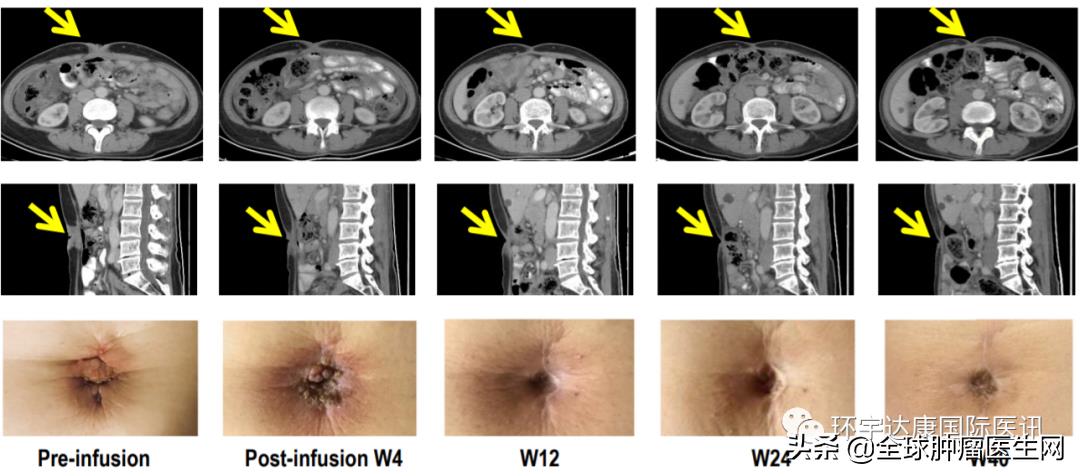
Source CARsgen Therapeutics
GC has peritoneal metastasis and Mary Joseph’s nodule (a nodular lesion formed by the metastasis of malignant tumor to the umbilical region). It has received 3-line therapy including PD-1 antibody and CLDN18.2 80%(2+) before, and achieved partial remission after CAR-T treatment, and continued to respond for more than 56 weeks.
03
Application of gucy2car-t in colorectal cancer
C(guanylyl cyclase C (GCC/GUCY2C) is a member of the receptor guanyl cyclase family, and it is the receptor of guanyl, guanyl and Escherichia coli enterotoxin, and plays a key role in the liquid and ion homeostasis of gastrointestinal tract. About 80% patients with colorectal cancer have positive expression of GCC. The expression of GCC in normal tissues is limited to the top of intestinal epithelial cells, but not in other tissues and organs. In recent years, it has been found that GCC is stably expressed in primary colorectal cancer cells, but abnormally highly expressed in metastatic colorectal cancer cells, which is considered as one of the specific marker molecules of metastatic colorectal cancer.
GCC19CART is an autologous CAR-T therapeutic product, which is the leading solid tumor therapy for the treatment of relapsed and refractory metastatic colorectal cancer (R/R mCRC). GCC19CART specifically targets and eliminates cancer cells that express colorectal cancer tumor marker GCC. According to public information, the clinical trial of 35 patients with relapsed and refractory colorectal cancer conducted by GCC19CART in China has achieved initial results. In the early clinical trial (IRB) initiated by China, the researchers enrolled 35 patients with advanced colorectal cancer. Among the 8 patients who received a dose of 2×106 GCC19CART cell/kg in the dose climbing test, an objective remission rate of 50% was observed.
On April 19th, 2022, Shanghai Stensai Bio announced that its solid tumor CAR-T product GCC19CART was granted fast-track qualification by the US Food and Drug Administration (FDA). Have to say, this honor makes China CAR-T therapy take another solid step on the road of overcoming solid tumor, which is worth looking forward to!
04
Mesothelin CAR-T therapy
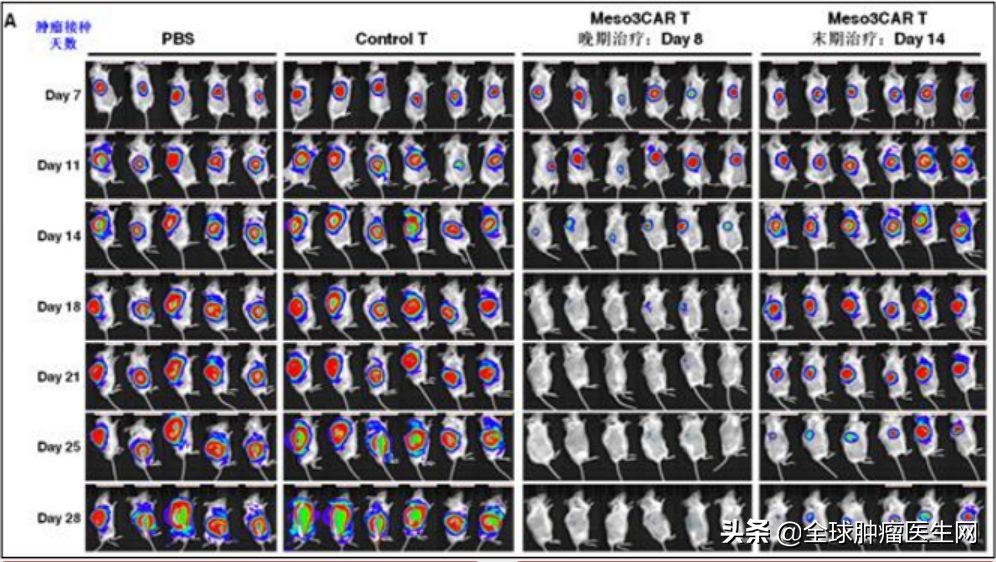
Mesothelin is a cell surface glycoprotein, which is highly expressed in many tumors, such as malignant pleural mesothelioma, pancreatic cancer, ovarian cancer and part of lung cancer, but poorly expressed in normal pleura, peritoneum and pericardium. Preclinical studies show that CAR-T cells targeting mesothelin have potential anti-tumor effects.
The latest results of mesothelin CAR-T therapy published by the University of Pennsylvania at the American Society of Clinical Oncology in 2019 showed that six patients with refractory metastatic pancreatic ductal adenocarcinoma were successfully enrolled, and all patients had received two or more treatments. These patients were infused with mesothelin CAR T cells three times a week for a total of nine doses. The results showed that there were 2 patients with stable diseases, and their progression-free survival time was 3.8 months and 5.4 months.
Therefore, this new therapy has biological activity in patients with pancreatic cancer, and this study is still in clinical trials (NCT03323944). At present, a large number of mesothelin CAR-T clinical studies have been carried out in China, and preliminary results have been achieved. In mouse experiments, the tumors of advanced ovarian cancer mice receiving the new mesothelin CAR-T therapy have rapidly subsided. At present, this technology is recruiting all kinds of patients with advanced solid tumors in China. Those who want to participate can apply to the Medical Department of the Global Oncologist Network.
Spring is blooming, and more new treatments are on the way!
Before the advent of cell therapy, solid tumors, including advanced gastric adenocarcinoma and pancreatic cancer, were usually treated by surgery, radiotherapy and chemotherapy. Among them, the incidence of gastric adenocarcinoma accounted for 95% of gastric malignant tumors, and pancreatic cancer was the most malignant tumor among common tumors. The median survival rate and 5-year survival rate were far lower than other tumors, so it was called "the king of cancer". However, most patients will have local recurrence or metastasis after operation. In addition, this kind of malignant tumor is not sensitive to radiotherapy and chemotherapy. Therefore, based on the current standard therapy, the treatment effect is not ideal, and the prognosis is extremely poor. The emergence of immunotherapy will bring hope and miracle for long-term survival to more advanced patients.
We firmly believe that after the introduction of a perfect cellular immune regulatory system, the country will open the door of cellular immunotherapy to benefit more cancer patients, and our country’s cellular immunotherapy will also enter the international stage!